 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!
 Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!
| 제품번호 | 종 | 제품 설명 | 구조 | 순도 | 특징 |
|---|---|---|---|---|---|
| PDB-C52H3 | Canine | Canine PDGF R beta / CD140b Protein, His Tag |  |
|
|
| PDB-H5259 | Human | Human PDGF R beta / CD140b Protein, Fc Tag |  |
|
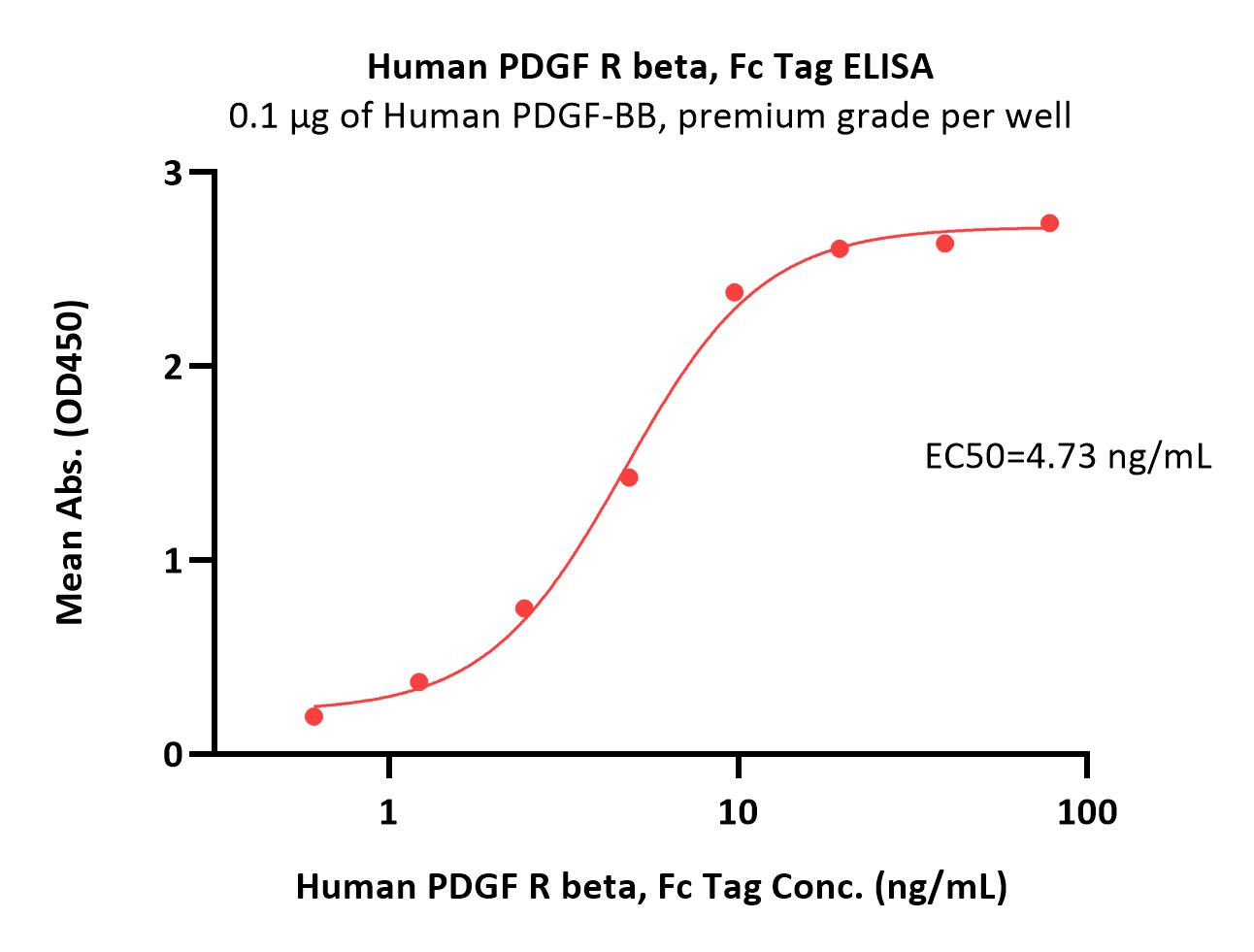
Immobilized Human PDGF-BB, premium grade (Cat. No. PDB-H4112) at 1 μg/mL (100 μL/well) can bind Human PDGF R beta, Fc Tag (Cat. No. PDB-H5259) with a linear range of 0.6-10 ng/mL (QC tested).
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Becaplermin biosimilar (Virchow Group) | Approved | Virchow Group | Healace, Plermin gel | Diabetic Foot | Details | |||||
| Becaplermin | RWJ-60235; rhPDGF-BB (Janssen) | Approved | Janssen Global Services Llc | Regranex | United States | Ulcer; Lower extremity diabetic neuropathic ulcers | Smith & Nephew Healthcare Ltd | 1997-12-16 | Diabetic Foot; Diabetic Neuropathies; Lower extremity diabetic neuropathic ulcers; Skin Ulcer; Foot Ulcer; Parkinson Disease; Ulcer; Varicose Ulcer | Details |
| Midostaurin | PKC-412; PKC-412A; CGP-41231; CGP-41251 | Approved | Novartis Pharma Ag | Rydapt | EU | Leukemia, Myeloid, Acute; Mastocytosis | Novartis Europharm Ltd | 2017-04-28 | Hematologic Neoplasms; Leukemia; Mastocytosis, Systemic; Myelodysplastic Syndromes; Leukemia, Mast-Cell; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Hepatic Insufficiency; Leukemia, Myeloid, Acute; Mastocytosis | Details |
| Nintedanib Esylate | BIBF-1120 | Approved | C.H. Boehringer Sohn Ag & Co. Kg | Ofev, Vargatef | EU | systemic sclerosis-associated interstitial lung disease | Boehringer Ingelheim International Gmbh | 2014-10-15 | Gliosarcoma; Breast Neoplasms; Sarcoma; systemic sclerosis-associated interstitial lung disease; Adenocarcinoma, Clear Cell; Peritoneal Neoplasms; Colorectal Neoplasms; Astrocytoma; Hepatic Insufficiency; Genital Neoplasms, Female; Silicosis; Prostatic Neoplasms; Carcinoma, Squamous Cell; Appendiceal Neoplasms; Fallopian Tube Neoplasms; Leukemia, Myeloid, Acute; Uterine Neoplasms; Endometrial Neoplasms; Carcinoma, Non-Small-Cell Lung; Adenocarcinoma; Carcinoma, Endometrioid; Carcinoma, Hepatocellular; Colonic Neoplasms; Ovarian Neoplasms; Telangiectasia, Hereditary Hemorrhagic; Esophageal Neoplasms; Rejection of lung transplantation; Carcinoid Tumor; Endometrial Stromal Tumors; Carcinoma, Renal Cell; Radiation Pneumonitis; Neoplasms; Idiopathic Pulmonary Fibrosis; Solid tumours; Scleroderma, Systemic; Glioblastoma; Small Cell Lung Carcinoma; Mesothelioma; Neuroendocrine Tumors; Pulmonary Fibrosis; Lung Diseases, Interstitial; Multiple Myeloma; Asbestosis; Oligodendroglioma | Details |
| Regorafenib | DAST; BAY-73-4506 | Approved | Bayer AG | Stivarga, Resihance | Mainland China | Gastrointestinal Stromal Tumors | Bayer Pharma Ag | 2012-09-27 | Leukemia, Myeloid, Acute; Sarcoma; Gastrinoma; Osteosarcoma; Sarcoma, Ewing; Colorectal Neoplasms; Peritoneal Neoplasms; Gastrointestinal Stromal Tumors; Bile Duct Neoplasms; Fallopian Tube Neoplasms; Esophageal adenocarcinoma; Adenoma; Thyroid Neoplasms; Lung Neoplasms; Thymoma; Carcinoma, Hepatocellular; Adenocarcinoma; Melanoma; Neoplasm Metastasis; Gastrointestinal Neoplasms; Somatostatinoma; Carcinoma, Islet Cell; Ovarian Neoplasms; Liver Neoplasms; Hemangiosarcoma; Esophageal Neoplasms; Stomach Neoplasms; Carcinoma, Renal Cell; Rectal Neoplasms; Carcinoid Tumor; Insulinoma; Solid tumours; Colonic Neoplasms; Neoplasms; Carcinoma, Ovarian Epithelial; Pancreatic Neoplasms; Small Cell Lung Carcinoma; Glioblastoma; Carcinoma, Transitional Cell; Carcinoma, Adenoid Cystic; Glucagonoma | Details |
| Anlotinib Dihydrochloride | AL-3818 | Approved | Advenchen Laboratories Llc, Jiangsu Chia Tai-Tianqing Pharmaceutical Co Ltd | 福可维 | Mainland China | Thyroid Neoplasms | Jiangsu Chia Tai-Tianqing Pharmaceutical Co Ltd | 2018-05-08 | Esophageal Squamous Cell Carcinoma; Bone Neoplasms; Peritoneal Neoplasms; Colorectal Neoplasms; Hepatic Insufficiency; Gastrointestinal Stromal Tumors; Urologic Neoplasms; Sarcoma, Alveolar Soft Part; Endometrial Neoplasms; Gallbladder Neoplasms; Bile Duct Diseases; Thyroid Neoplasms; Fallopian Tube Neoplasms; Lung Neoplasms; Glioma; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms; Melanoma; Carcinoma, Hepatocellular; Osteoma; Small Cell Lung Carcinoma; Head and Neck Neoplasms; Solid tumours; Biliary Tract Neoplasms; Drug-Related Side Effects and Adverse Reactions; Ovarian Neoplasms; Esophageal Neoplasms; Thoracic Neoplasms; Carcinoma, Renal Cell; Stomach Neoplasms; Leiomyosarcoma; Lymphoma, Large B-Cell, Diffuse; Neoplasms; Carcinoma, Ovarian Epithelial; Sarcoma, Synovial; Neuroendocrine Tumors; Liver Diseases; Sarcoma; Medullary thyroid cancer (MTC); Nasopharyngeal Carcinoma | Details |
| Sorafenib Tosylate | NSC-724772; BAY-43-0006; BAY-43-9006; BAY-54-9085 | Approved | Onyx Pharmaceuticals Inc | Nexavar, 多吉美 | EU | Thyroid Neoplasms | Bayer AG | 2005-12-01 | Liver Neoplasms; Leukemia, Myeloid; Lymphoma, B-Cell, Marginal Zone; Rhabdomyosarcoma; Head and Neck Neoplasms; Leukemia, Myelogenous, Chronic, BCR-ABL Positive; Fibromatosis, Aggressive; Leiomyosarcoma; Kidney Neoplasms; Leukemia, Erythroblastic, Acute; Solid tumours; Lymphoma, T-Cell, Peripheral; Ovarian Neoplasms; Recurrence; Carcinoma, Renal Cell; Esophageal Neoplasms; Vipoma; Rectal Neoplasms; Squamous Cell Carcinoma of Head and Neck; Histiocytoma, Malignant Fibrous; Carcinoma, Islet Cell; Hemangiosarcoma; Carcinoid Tumor; Carcinoma; Insulinoma; Stomach Neoplasms; Neoplasms; Pancreatic Neoplasms; Multiple Endocrine Neoplasia Type 2a; Hypertension, Portal; Carcinoma, Transitional Cell; Carcinoma, Verrucous; Thyroid Carcinoma, Anaplastic; Myelodysplastic Syndromes; Glioblastoma; Carcinoma, Ovarian Epithelial; Leukemia, Myelomonocytic, Acute; Colonic Neoplasms; Lymphoma, Large B-Cell, Diffuse; Lymphomatoid Granulomatosis; Wilms Tumor; Leukemia, Myelomonocytic, Chronic; Lymphoma, Large-Cell, Immunoblastic; S | Details |
| Dasatinib Hydrate | BMS-354825; NSC-732517 | Approved | Bristol-Myers Squibb Company | 施达赛, Spricel, Sprycel, Spraysel | Mainland China | Leukemia, Myelogenous, Chronic, BCR-ABL Positive; Precursor Cell Lymphoblastic Leukemia-Lymphoma | Bristol-Myers Squibb Pharma Eeig | 2006-06-28 | Astrocytoma; Intraocular Lymphoma; Lymphoma, Large-Cell, Anaplastic; Lymphoma, T-Cell; Lymphoma, T-Cell, Cutaneous; Lymphoma, Non-Hodgkin; Fallopian Tube Neoplasms; Lung Neoplasms; Gastrointestinal Stromal Tumors; Giant Cell Tumor of Bone; Primary Myelofibrosis; Gliosarcoma; Burkitt Lymphoma; Peritoneal Neoplasms; Leukemia, Myeloid, Chronic-Phase; Sarcoma, Ewing; Neurofibrosarcoma; Sarcoma; Cholangiocarcinoma; Brain Neoplasms; Precursor T-Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Mantle-Cell; Lymphoma, Follicular; Prostatic Neoplasms; Neoplasm Metastasis; Mastocytosis; Tongue Neoplasms; Carcinoma, Hepatocellular; Adenocarcinoma; Carcinoma, Non-Small-Cell Lung; Leukemia, Large Granular Lymphocytic; Leukemia, T-Cell; Leukemia, Lymphocytic, Chronic, B-Cell; Mycosis Fungoides; Melanoma; Heart Arrest; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Endometrial Neoplasms; Leukemia, Myeloid, Acute; Hemangiopericytoma; Sarcoma, Alveolar Soft Part; Uterine Neoplasms; Laryngeal Neoplasms; Waldenstrom Macroglobuli | Details |
| Pazopanib Hydrochloride | GSK-786034; GW-786034B; SB-786034; GW-786034 | Approved | Glaxosmithkline Plc, Novartis Pharma Ag | 维全特, Armala, Votrient, Patorma | Mainland China | Carcinoma, Renal Cell | Novartis Pharma Schweiz Ag | 2009-10-19 | Peritoneal Neoplasms; Lymphoma; Uterine Neoplasms; Lung Neoplasms; Carcinoma, Small Cell; Thyroid Neoplasms; Gastrointestinal Stromal Tumors; Gliosarcoma; Carcinoma, Mucoepidermoid; Genital Neoplasms, Female; Uterine Cervical Diseases; Choriocarcinoma; Colorectal Neoplasms; Neuroblastoma; Osteosarcoma; Gastrinoma; Psoriasis; Brain Neoplasms; Urethral Neoplasms; Breast Neoplasms; Medullary thyroid cancer (MTC); von Hippel-Lindau Disease; Gastrointestinal Neoplasms; Carcinoma, Hepatocellular; Melanoma; Endodermal Sinus Tumor; Neoplasms, Germ Cell and Embryonal; Paraganglioma; Thyroid Cancer, Papillary; Neoplasm Metastasis; Uterine Cervical Neoplasms; Breast Neoplasms, Male; Prostatic Neoplasms; Macular Degeneration; Carcinoma, Non-Small-Cell Lung; Fallopian Tube Neoplasms; Leukemia, Myeloid, Acute; Glioma; Germinoma; Carcinoma, Neuroendocrine; Carcinoma, Embryonal; Carcinoma, Squamous Cell; Telangiectasia, Hereditary Hemorrhagic; Thyroid Carcinoma, Anaplastic; Neoplasms; Carcinoid Tumor; Herpes Genitalis; Pheoc | Details |
| Sunitinib Malate | PHA-290940; SU-010398; SU-11248; PNU-290940AD; PHA-290940AD; GB-102; PNU-290940; SU-011248-L-malate salt | Approved | Pfizer Pharmaceuticals Ltd (China) | 索坦, Sutent | Japan | Pancreatic neuroendocrine tumors (pNET) | Pfizer Inc | 2006-01-26 | Teratoma; Ovarian Neoplasms; Fibromatosis, Aggressive; Solid tumours; Intestinal Neoplasms; Kidney Neoplasms; Liver Neoplasms; Leiomyosarcoma; Fibrosarcoma; HIV Infections; Head and Neck Neoplasms; Lymphoma, B-Cell, Marginal Zone; Lymphoma, B-Cell; Leukemia, Myeloid, Accelerated Phase; Leukemia; Leukemia, Myelogenous, Chronic, BCR-ABL Positive; Lymphoma, T-Cell, Peripheral; Ependymoma; Carcinoma, Renal Cell; Histiocytoma, Malignant Fibrous; Carcinoma; Hemangioblastoma; Carcinoma, Islet Cell; Stomach Neoplasms; Squamous Cell Carcinoma of Head and Neck; Thoracic Neoplasms; Leukemia, Hairy Cell; Abdominal Neoplasms; Esophageal Neoplasms; Polycythemia Vera; Pelvic Neoplasms; Pheochromocytoma; Glioblastoma; Neurofibromatoses; Pancreatic Neoplasms; Pancreatic neuroendocrine tumors (pNET); Salivary Gland Neoplasms; Small Cell Lung Carcinoma; Leukemia, Myelomonocytic, Chronic; Myelodysplastic Syndromes; Hodgkin Disease; Lymphoma, Large B-Cell, Diffuse; Carcinoma, Ovarian Epithelial; Carcinoma, Papillary; Neoplasms; I | Details |
| Ripretinib | DCC-2618 | Approved | Deciphera | 擎乐, Qinlock | United Kingdom | Gastrointestinal Stromal Tumors | Deciphera Pharmaceuticals Llc | 2020-05-15 | Mastocytosis, Systemic; Neoplasms; Gastrointestinal Stromal Tumors | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Sorafenib Tosylate/MG-K10 | MG-D-1609 | Phase 2 Clinical | Solid tumours | Details | |
| R-1530 | RG1530; R-1530; RG-1530 | F. Hoffmann-La Roche Ltd | Details | ||
| CLS-1002 | CLS-1002 | Phase 1 Clinical | Clearside Biomedical Inc | Macular Degeneration | Details |
| ZSP-1603 | ZSP-1603 | Phase 2 Clinical | Wuxi Apptec Co Ltd, Guangdong Zhongsheng Pharmaceutical Co Ltd | Ovarian Neoplasms; Liver Neoplasms; Solid tumours; Carcinoma, Renal Cell; Colonic Neoplasms; Idiopathic Pulmonary Fibrosis; Thyroid Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| Tafetinib Malate | SIM-0702; SIM-1005; SIM-010603 | Phase 1 Clinical | Jiangsu Simcere Pharmaceutical Co Ltd, Nanjing Yoko Biomedical Co Ltd, Jilin Boda Pharmaceutical Co Ltd | Neoplasms | Details |
| Crenolanib Besylate | CP-868596-26; IND-112201; CP-868596; ARO-002; ARO-002-26 | Phase 3 Clinical | Pfizer Pharmaceuticals Ltd (China) | Gastrointestinal Stromal Tumors; Leukemia, Myeloid, Acute; Glioma | Details |
| 18F-Dasatinib | 18F-SKI-249380; [18F]-Fluoro-BMS-354825; [18F]Dasatinib; [18F]SKI-249380; [18F]Fluoro-BMS-354825 | Phase 1 Clinical | Memorial Sloan Kettering Cancer Center | Bone Marrow Neoplasms; Carcinoma; Neoplasms; Sarcoma; Diagnostic agents; Lymphoma; Melanoma | Details |
| Lucitanib | AL-3810; CO-3810; S-80881 | Phase 3 Clinical | Advenchen Laboratories Nanjing Ltd | Solid tumours; Stomach Neoplasms; Small Cell Lung Carcinoma; Breast Neoplasms; Nasopharyngeal Carcinoma; Colorectal Neoplasms; Genital Neoplasms, Female; Thymus Neoplasms; Carcinoma, Small Cell; Lung Neoplasms; Carcinoma, Non-Small-Cell Lung; Carcinoma, Hepatocellular | Details |
| KDR2-2 | Phase 2 Clinical | Guangzhou Huiborui Biomedical Technology Co Ltd | Glaucoma, Neovascular; Neovascularization, Pathologic; Corneal Neovascularization | Details | |
| Metarafenib | Phase 1 Clinical | Guangzhou Nanxin Pharma Co Ltd | Solid tumours; Liver Neoplasms | Details | |
| Telatinib | EOC-315; BAY-57-9352 | Phase 2 Clinical | Bayer AG | Solid tumours; Stomach Neoplasms | Details |
This web search service is supported by Google Inc.
